Research Coordinator
PICU
Children's Hospital of Easter Ontario Research Institute
Quality improvement tools to manage deceased organ donation processes: a scoping review
Amina Silva1,3, Sonny Dhanani1, Laura Hornby4, Marian Luctkar-Flude3, Andrea Rochon3, Ken Lotherington4, Lindsay Wilson4, Samantha Arora4, Vanessa Silva e Silva2.
1PICU, CHEO-RI, Ottawa, ON, Canada; 2Nursing Department, Brock University, St Catherines, ON, Canada; 3School of Nursing, Queens' University, Kingston, ON, Canada; 4Canadian Blood Services, Canadian Blood Services, Ottawa, ON, Canada
Background: Deceased organ donation, both after circulatory determination of death (DCD) and after neurological determination of death (NDD), is a highly complex and multi-phased process. To ensure a proper flow of the donation process, appropriate quality improvement tools should be used. These tools allow better control over health activities with more reliable and predictable outcomes. However, there is still a paucity of comprehensive evidence about the use of these tools to manage deceased donation processes. Therefore, this scoping review aims to summarize the international literature on quality improvement tools developed to manage deceased organ donation processes (DCD/NDD).
Methods: Scoping review using the JBI methodology. Published literature was searched on MEDLINE, Embase, PsycINFO, CINAHL, Web of Science and Academic Search Complete from inception to July 2021 (updated in June, 2022). Unpublished and gray literature included reports from organ donation organizations. Reports were considered if they described the use of quality improvement tools to manage deceased donation processes in any healthcare setting. Data were screened, extracted and analyzed by two independent reviewers.
Results: The first search yielded over 10000 citations and 40 were included in this review. Most reports were written in English (n=38), from Canada (n=21), and published between 2016 and 2022 (n=22). The tools identified included checklists, algorithms, flow charts, charts, pathways, decision tree maps and mobile apps. These tools were applied in the following phases of the organ donation process: (1) potential donor identification, (2) donor referral, (3) donor assessment and risk, (4) donor management, (5) withdrawal of life-sustaining measures,(6) death determination, (7) organ retrieval and (8) overall organ donation process.
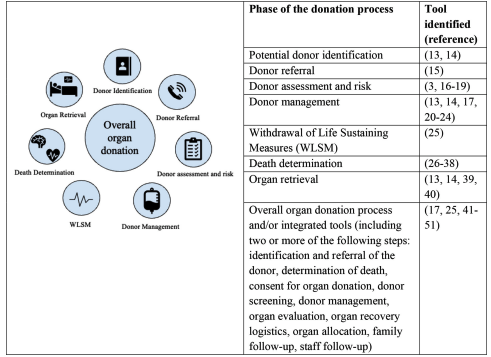
Conclusion: The existing evidence lacks details in the report of methods used for the development, testing and impact of these tools, and we could not locate tools specific to some phases of the organ donation process. Lastly, by mapping existing tools, we aim to facilitate both clinician choices among available tools, as well as research work building on existing knowledge.
The authors would like to acknowledge the Canadian Donation and Transplantation Research Programme (CDTRP), Canadian Blood Services (CBS) and Children’s Hospital of Eastern Ontario (CHEO) for all their support and guidance in the development of this research. We also thank Robin Featherstone, Cochrane Information Specialist, for developing and employing the main electronic search strategies, and Amanda Ross-White, from JBI Centre of Excellence, for peer-reviewing the search strategy.
Lectures by Amina R Silva
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Thu-19 13:00 - 14:00 |
DCD | Quality improvement tools to manage deceased organ donation processes: a scoping review | South Seas Ballroom A/B |
|
Thu-19 13:00 - 14:00 |
Donor Detection | Quality improvement tools to manage organ donation processes: an instrumental case study | South Seas Ballroom A/B |
