Daniel H. Drake, M.D. graduated from the University of Michigan Medical School in 1982. Following a general surgery residency in Texas he returned to the University of Michigan for a fellowship in Thoracic Surgery. Dr. Drake co-founded Munson Medical Center’s cardiothoracic surgery (CTS) program in 1990 and practiced in the Cardiothoracic Surgeons of Grand Traverse for 28 years. Dr. Drake served as President of the Michigan Society of Thoracic & Cardiovascular Surgeons from 1999 until 2001. He speaks nationally and internationally on image-guidance for mitral disease. He is a member of the American Society of Echocardiography Council on Perioperative Echocardiography and editorial board of the American Society of Echocardiography journal CASE. Dr. Drake serves on the Board of the Society of Thoracic Surgeons Standards and Ethics Committee and is a member of the Cardiothoracic Ethics Forum. After a long clinical career focused on cardiac valve imaging and complex mitral reconstruction, Dr. Drake transitioned to laboratory translational research and teaching. He prepared for this transition by spending two years as a research fellow in the University of Michigan Extracorporeal Life Support (ECLS) Laboratory under the mentorship of Professor Emeritus Robert H. Bartlett. His research focus is on extracorporeal life support, organ banking and nitric oxide. During the Covid-19 pandemic, Dr. Drake relocated to the National Institute for Allergy and Infectious Diseases Laboratory in Columbia, Missouri where he facilitated the development of an aerosolized SARS-CoV-2 model of pneumonia for testing the anti-viral efficacy of nitric oxide. He is supported by NIH funding and other grants.
Evaluating cardiac viability before transplantation: 24-hour normothermic ex vivo heart perfusion of the left atrium
Brianna Spencer1, Spencer Wilhelm1, Kristopher Urrea1, Sebastian Sewera1, Vikramjit Chakrabortty1, Jamie Ho1, Tahmina Sultana1, Daniel Mazur1, Robert H Bartlett1, Alvaro Rojas Pena1,2, Daniel D Drake1,3.
1Surgery, Extracorporeal Life Support Laboratory, University of Michigan, Ann Arbor, MI, United States; 2Surgery, Section of Transplantation, University of Michigan, Ann arbor, MI, United States; 3Cardiac Surgery, University of Michigan, Ann Arbor, MI, United States
Introduction: Cold static storage (5ºC) or normothermic (37ºC) ex vivo heart perfusion (NEVHP) in the beating but resting mode (Langendorff) successfully preserve hearts for transplantation for up to 6 hours. Previous attempts to prolong the preservation period have increased morbidity and mortality following transplantation. In addition, the 6-hour limit imposes geographic restrictions for recipients, prevents optimal organ assessment, and reduces the number of hearts available for transplantation. Our group successfully maintained 10 consecutive piglet hearts (60±8.8 g) for 24 hours using NEVHP with hemofiltration in the beating, resting-heart mode. Although this prolonged NEVHP model was successful, cardiac function could not be comprehensively assessed in the beating, resting-heart mode. Therefore, this work aimed to expand our prolonged NEVHP model to include intermittent left atrial (LA) perfusion that would allow cardiac assessment in the working-heart mode.
Methods: Following anesthetic induction, sternotomy, cardioplegia administration, explantation, and back-table instrumentation,
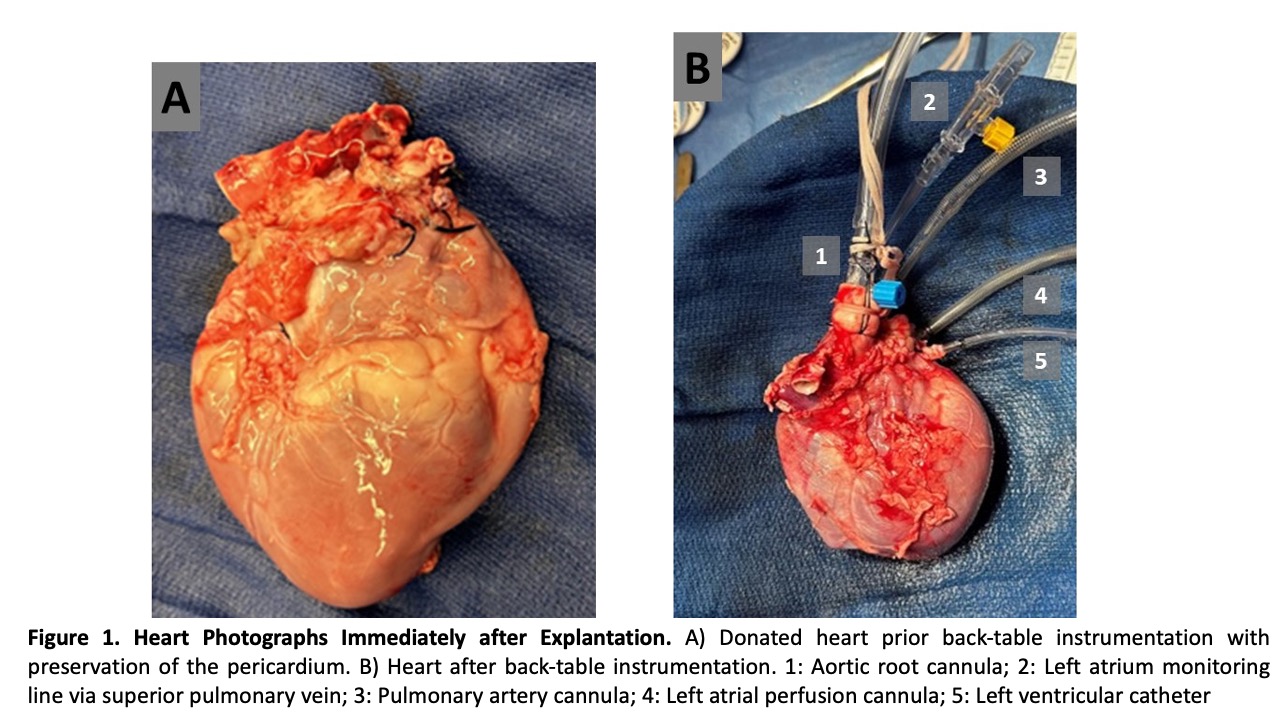
NEVHP was initiated in beating, resting mode in 9 hearts. After 1 hour the circuit was transitioned to LA perfusion working-heart mode for 30 minutes, baseline working-heart parameters were documented and perfusion was returned to beating, resting-heart mode. Intermittent LA perfusion with working-heart assessment was performed every 4-6 hours. Final working-heart measurements were obtained at 24 hours. After 24 hours NEVHP one orthotopic heart transplant was planned in a healthy recipient.
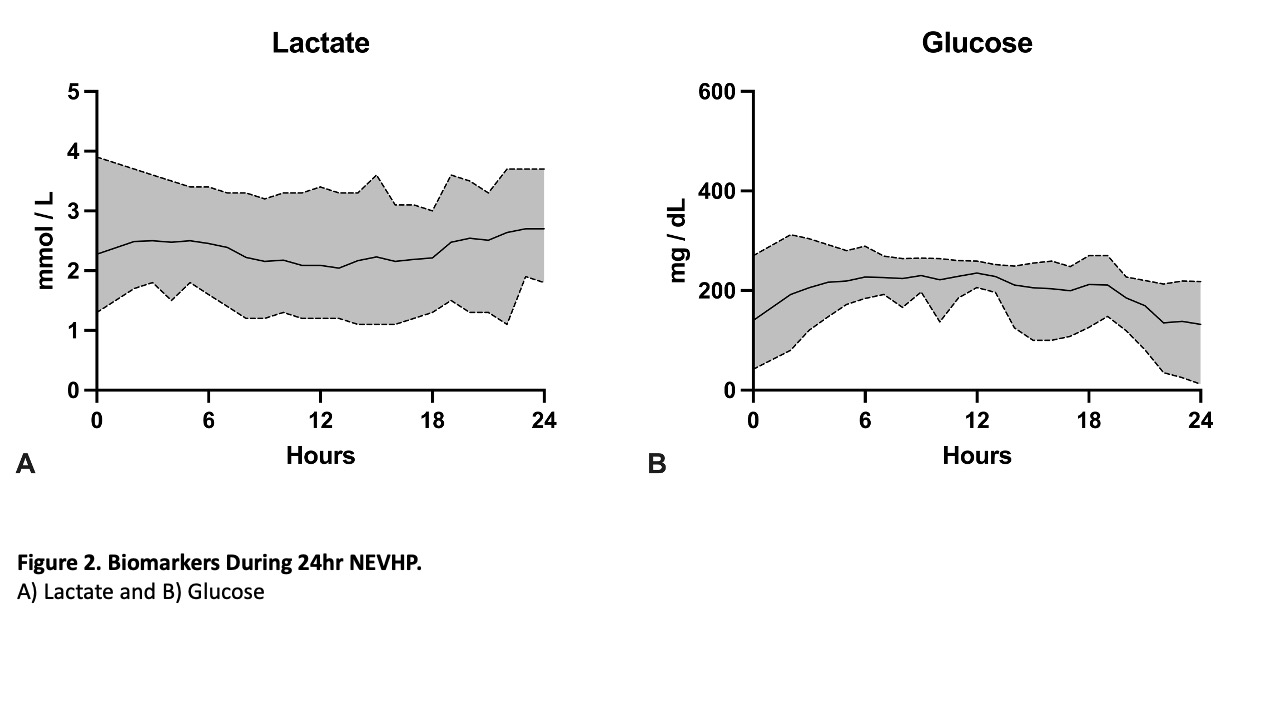
Results: 24-hour NEVHP on 9 consecutive hearts (280±42.1 g) was successful and all hearts maintained working-heart parameters without difference compared to baseline. Mean perfusion pressures were: LA= 5.6±2.6 mmHg; Left ventricle (LV)= 18.0±6.3 mmHg; and aortic root= 27.4±8.5 mmHg. Coronary flow was maintained at 0.59±0.1 mL/min/g with a coronary vascular resistance of 169.6±58.2 mmHg/mL/min. Oxygen consumption was 0.91±0.5 mL/min/g with an oxygen extraction ratio of 12.3%±5.7%. Lactate levels were 2.3±0.7 mmol/L.
The transplanted heart recipient was weaned off cardiopulmonary bypass for one hour.
Conclusions: This work demonstrates the successful development and validation of prolonged (24-hour) NEVHP in a porcine model with the assessment of cardiac function in the working-heart mode using intermittent LA perfusion. Intermittent LA perfusion is an important step toward a comprehensive ex-vivo assessment of donor heart function. In addition, successful orthotopic heart transplantation after 24 hours in a heart with stable LA perfusion parameters corroborates our methods. This is an essential step toward clinical usage of prolonged NEVHP and real-time heart assessment for future heart transplantation.
Maxine and Stuart Frankel Foundation. Frankel Innovation Initiative - University of Michigan.