Leonie van Leeuwen, Netherlands has been granted the TTS Congress Scientific Award
Targeted delivery of galunisertib using machine perfusion reduces fibrogenesis in an integrated ex vivo renal transplant and fibrogenesis model
Leonie van Leeuwen1,2, Mitchel J.R. Ruigrok3, Chris L Jaynes1,4, Benedikt M. Kessler2, Henri G.D. Leuvenink1, Peter Olinga3.
1Department of Surgery, University Medical Center Groningen, Groningen, Netherlands; 2Nuffield Department of Medicine, Centre for Medicines Discovery, Target Discovery Institute, University of Oxford, Oxford, United Kingdom; 3Department of Pharmaceutical Technology and Biopharmacy, Groningen Research Institute of Pharmacy, University of Groningen, Groningen, Netherlands; 434 Lives, West Lafayette, IN, United States
Introduction: Yearly, ten thousand kidney grafts are discarded in the United States alone. One approach to decrease this discard rate is the implementation of normothermic machine perfusion. This technique could be improved by adding therapeutics—such as antifibrotic molecules—to perfusion solutions, hereby exploring targeted drug delivery to an isolated organ whilst eliminating the risk of systemic adverse effects. We introduce a novel approach to suppress fibrosis, one of the major post-transplant complications that contribute to graft failure. Our approach involves 6 hours of ex vivo perfusion of porcine kidneys with a blood-based perfusate spiked with transforming growth factor beta (TGF-β) —one of the most important cytokines involved in fibrogenesis—and added galunisertib—a potent inhibitor of the TGF-β signaling pathway.
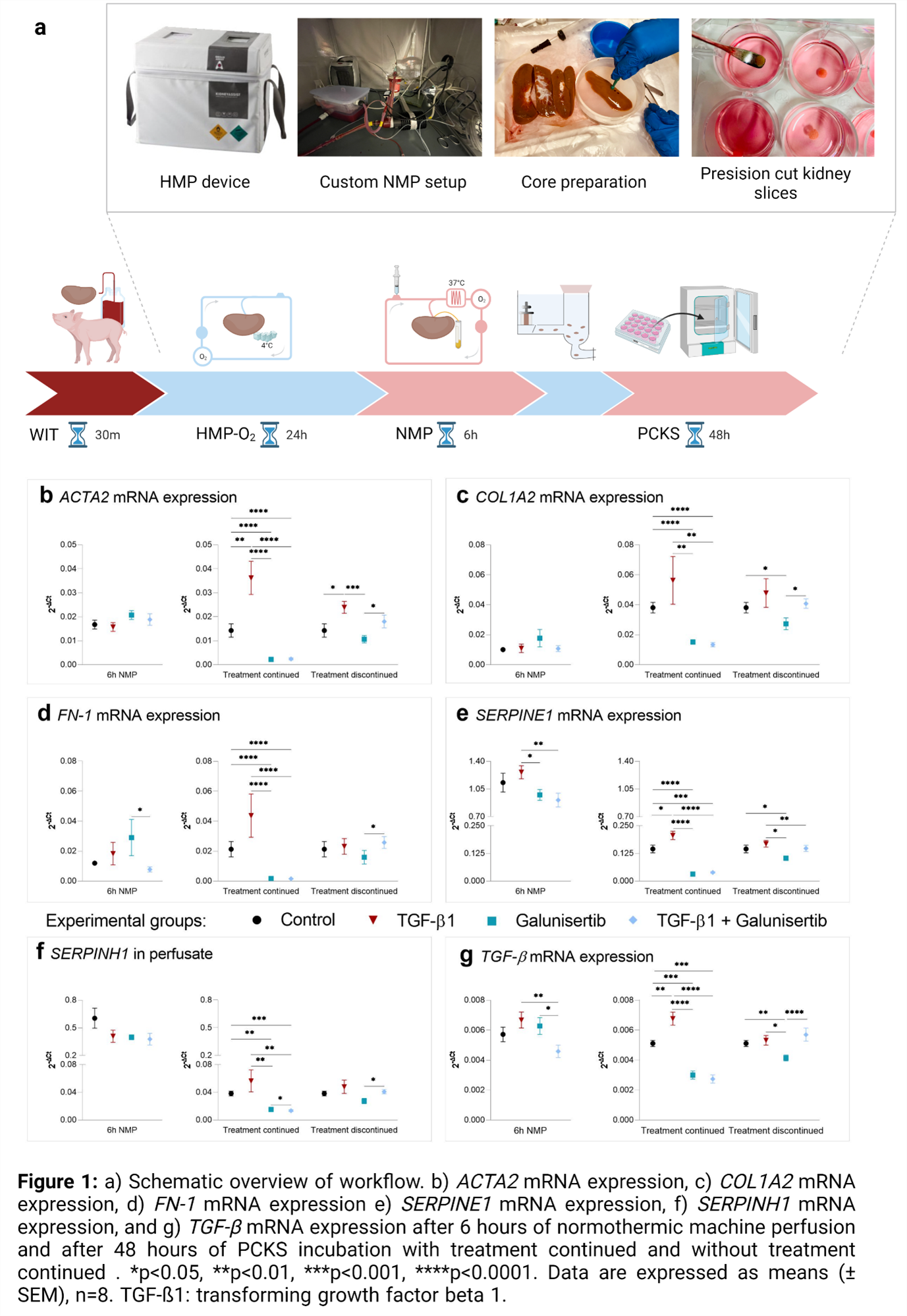
Method: Porcine kidneys were subjected to 30 minutes of warm ischemia, 24 hours of oxygenated hypothermic machine perfusion, and 6 hours of normothermic machine perfusion with various treatments (i.e., untreated control, TFG-β, galunisertib, or TGF-β+galunisertib; n=8 kidneys per group). To determine whether effects persisted upon ceasing treatment, kidney slices were prepared from respective kidneys and incubated for 48 hours (Figure 1a).
Results: Galunisertib supplementation improved the general viability, characterized by increased oxygen consumption, elevated ATP levels, and attenuated tubular dilation and necrosis. No significant differences in renal function, oxidative stress levels, or injury markers were observed. Galunisertib altered inflammation markers by causing a significant increase in gene expression of TNF-α, and a significant decrease of IL-6 after 6h NMP. This was supported by IL-6 protein expression. Continued TGF-β supplementation promoted fibrogenesis as shown by significantly increased mRNA expression of TGF-β, ACTA2, COL1A2, FN-1, SERPINE1, and SERPINH1 after 48 hours of incubation. Continued treatment with galunisertib, however, clearly attenuated the expression of all tested fibrosis-related genes after 48h incubation (Figure b-g).
Conclusion: Our findings suggest that galunisertib positively affected mitochondrial activity, tissue integrity and, most importantly, attenuated the expression of fibrogenesis-related genes. Therefore, galunisertib appears to be a promising drug for further research. Our next step is to evaluate the antifibrotic potential of galunisertib in human discarded kidneys using machine perfusion and tissue slicing. In conclusion, these findings illustrate the value of targeted delivery of galunisertib, using isolated organ perfusion, for reducing post-transplant complications and ultimately, limiting the organ discard rate.